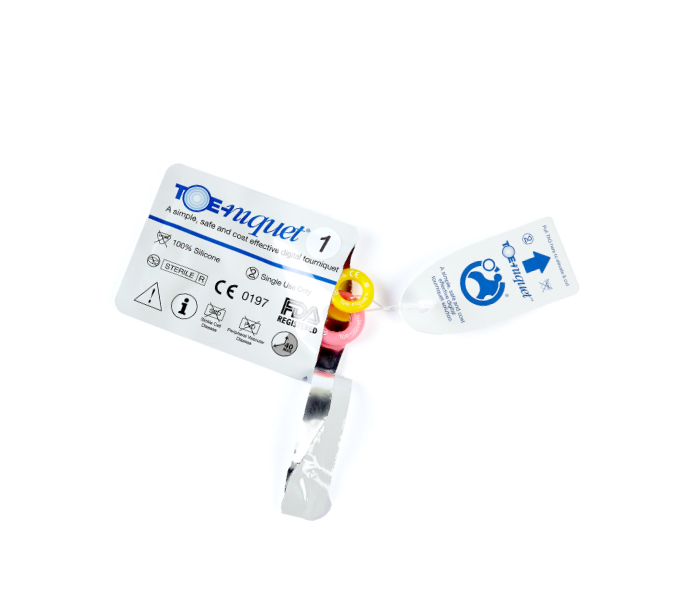
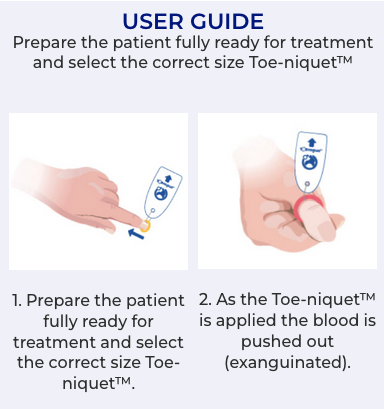
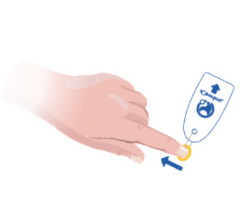
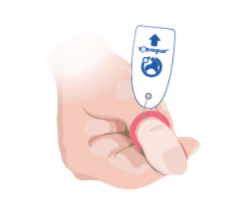
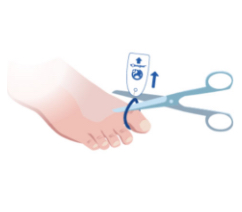
Toe-niquets™ are Single Use disposable ‘elastic-ring’ tourniquet devices designed to stop blood flow in digits of the hands and feet (fingers and toes) for use during surgery, trauma or first aid. Toe-niquets™ are easily applied by rolling over the tip of any digit.
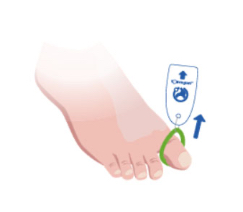
Toe-niquets™ allow you to exanguinate (remove) blood from a digit and stop further blood flow at the point of application. Treatment may continue safely, quickly and easily without the risks of visual or physical obstructions associated with bleeding.
Toe-niquets™ are used during surgery, trauma or first aid by Medical Doctors, Surgeons, Surgical Theatres, Emergency Room Facilities and Podiatrists worldwide. They are supplied sterile ready for use in any clinical or surgical environment.
Easy Use – Sterile Packaging

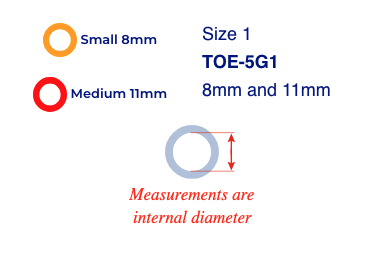
Size 1
TOE-5G1
8mm and 11mm
Size 2
TOE-5G2
14mm and 17mm





CONFORMITY TO APPROVED CLINICAL STANDARDS
Toe-niquets™ are individually packed, sterilised by irradiation and offer a 5 year shelf life. The peel pouch is made from high quality Tyvek and conforms to BSEN-5 1999 Standard, providing full traceability with batch numbers for
medical records.
Toe-niquets™ carry the ‘Single Use’’ only symbol for improved patient safety.
Toe-niquets™ conform to current EC Medical Devices Directive (93/42/EEC Annex V).
Toe-niquets™ conform to current FDA Directives.
Toe-niquets™ are CE Sterile and carry the CE Class 1 – Sterile Mark.
Toe-niquets™ are FDA Sterile registered and carry a FDA Registered Mark.
Toe-niquets™ meet the criteria and recommendations set out in the UK NHS National Patient Safety Agency’s, Rapid Response Report (NPSA/2007/RRR007 dated on 9th December, 2009) aimed at ‘Reducing risks of tourniquets left on after finger and toe surgery’
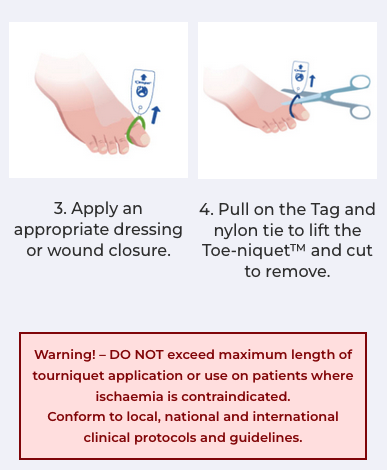
Conform to local, national and international clinical protocols and guidelines.
Sterility
Toe-niquets™ are individually packed,
sterilised by irradiation and offer a 5
year shelf life. The peel pouch is made
from high quality Tyvek and conforms
to BSEN-5 1999 Standard, providing full
traceability with batch numbers for
medical records.
Toe-niquets™ carry the ‘Single Use’’
only symbol for improved patient
safety.
Regulatory Standards
Toe-niquets™ conform to current EC Medical Devices Directive (93/42/EEC Annex V).
Toe-niquets™ conform to current FDA Directives.
Toe-niquets™ are CE Sterile and carry the CE Class 1 – Sterile Mark.
Toe-niquets™ are FDA Sterile registered and carry a FDA Registered Mark.
Health Services Standards
Toe-niquets™ meet the criteria and recommendations set out in the UK NHS National Patient Safety Agency’s, Rapid Response Report (NPSA/2007/RRR007 dated on 9th December, 2009) aimed at ‘Reducing risks of tourniquets left on after finger and toe surgery’

Pull Tag, Hypoallergenic
4 sizes (S, M, L, XL)
2 sets (S&M, L&XL)
Colour Coding & Strong
Optimal Elasticity Vs Compression Ratio
Latex free Medical Grade Silicone
Increased intra operative options
Less Stock for Distributors and Users
Improved Safety & Easier to Removal
Easy application, reduced tissue trauma